The DNA damage response to double strand breaks
Introduction
DNA double-strand breaks (DSBs) are constantly threatening genome integrity and must be repaired to prevent death, oncogenic transformation or ageing of cells. A vertebrate cell that sustains DSBs has to decide whether it sacrifices itself by apoptosis (see below) in order to protect the health of the organism or whether it tries to repair the damage. In the latter case, the cell then has to choose between two major repair pathways, the fast but potentially mutagenic NHEJ (non-homologous end joining) or the slower but error-free HR (homologous recombination). While NHEJ is hallmarked by rapid ligation of (tethered) DNA ends, HR involves DNA resection to generate long 3' single-stranded overhangs, invasion of the undamaged sister chromatid and primed DNA synthesis.
Again, despite great, recent advances in our knowledge, many questions about the cell's response to DNA damage remain to be elucidated. For example, it is clear that the key decisive event in repair pathway choice is whether or not DSB ends are resected. But exactly how the balance is tipped towards NHEJ or HR remains largely enigmatic. Interestingly, DSBs trigger the de novo recruitment of cohesin and replication-independent enhancement of sister chromatid cohesion, which supposedly facilitates homology search during HR. However, this finding seems to be at odds with reports of yeast separase also being required for proper HR. Thus, the putative role of separase for DNA damage response needs clarification.
An unexpected role of human separase in HR
We investigated whether separase might play a role in the DNA damage response of human cells. Using NHEJ- and HR-specific reporter cell lines, we showed that siRNA-mediated depletion of separase leaves NHEJ unaffected but compromises HR. Consistently, human separase is recruited to DSBs in G2- but not G1-phase (when HR is not operative ) as revealed by immunofluorescence microscopy (IFM) and chromatin immunoprecipitation (ChIP). IP-Western analyses of cohesin and fluorescence microscopy of a chromatin associated separase activity sensor further demonstrated that separase becomes proteolytically active during HR but, importantly, only locally at/around DSBs. Simultaneous de novo recruitment and cleavage of cohesin will render it dynamic at DSBs and this might be necessary to keep the undamaged sister chromatid close by but, at the same time, grant repair factors unimpaired access.
Separase contains an active nuclear export signal (NES) and is usually restricted to the cytoplasm in interphase. How then does it get into the nucleus during DDR to begin with? Furthermore, the active protease does not seem to diffuse, thus raising the question of how it is tethered to the sites of damage? Numerous experiments that were inspired by known recruitment mechanisms of established DDR factors led to the following answers: In response to DSBs, separase's NES is inactivated by phosphorylation of Ser1660. In addition, Lys1034 is sumoylated and an RG-repeat motif (centered around position 1426) is Arg-methylated. Preventing these PTMs by corresponding point mutations prevented separase from associating with damaged chromatin and to interact with the DNA damage marker gH2AX. Furthermore, cells that solely rely on these separase variants are compromised in their ability to recover from DSBs.
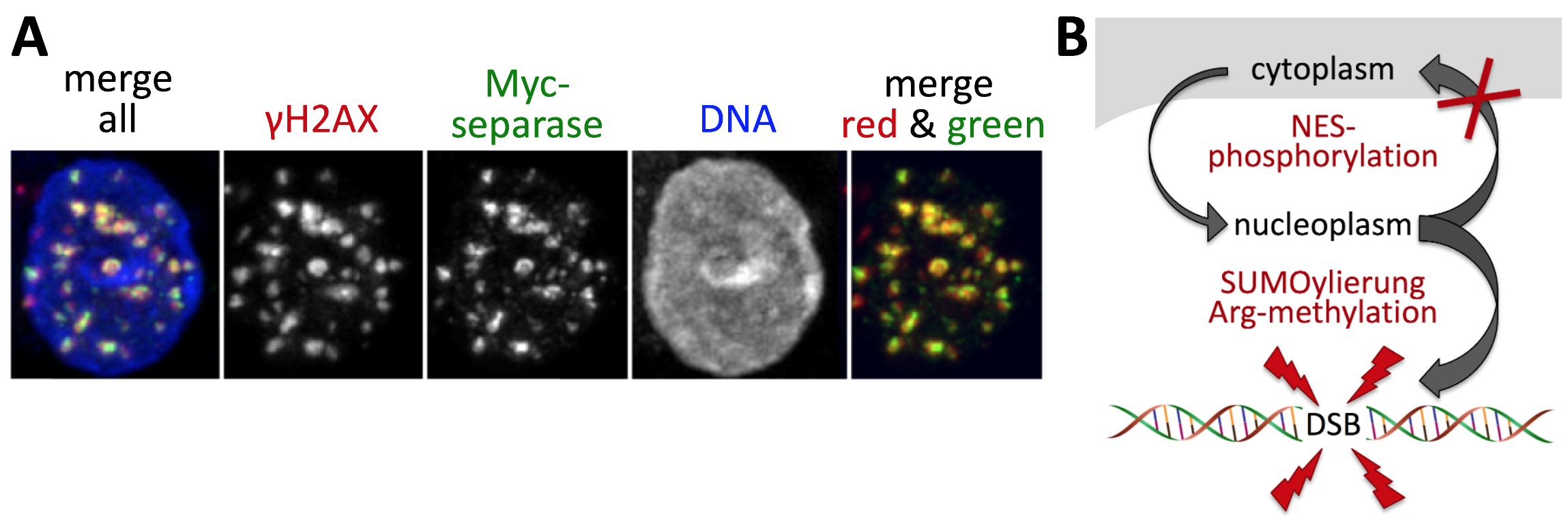
Fig. 3: Various PTMs direct separase to DSBs. A) Hek293 cells expressing Myc-tagged separase were doxorubicin-treated to induce DSBs and stained as indicated. B) Model of separase's PTM-dependent recruitment from the cytoplasm to DSBs.
Mice that are heterozygous for SEPARASE are much more prone to chemically induced skin cancer than the corresponding wild type animals. Consistently, SEPARASE+/- MEFs are hypersensitive to neoplastic transformation. The same SEPARASE+/- MEFs do not suffer from any measurable increase in aneuploidies or lagging chromosomes in anaphase. They do, however, exhibit reduced DSB-induced Rad21/cohesin cleavage and retarded DSB repair relative to wild type MEFs. Thus, separase is haploinsufficient in HR but not chromosome segregation. These observations further suggest that separase's function in DNA damage repair might help to prevent oncogenic transformation.
